Assessing Pantanal fauna through environmental DNA metabarcoding after the 2020 megafire
DOI:
https://doi.org/10.37002/biodiversidadebrasileira.v14i4.2558Palavras-chave:
Biodiversity , species monitoring, conservation unit, eDNAResumo
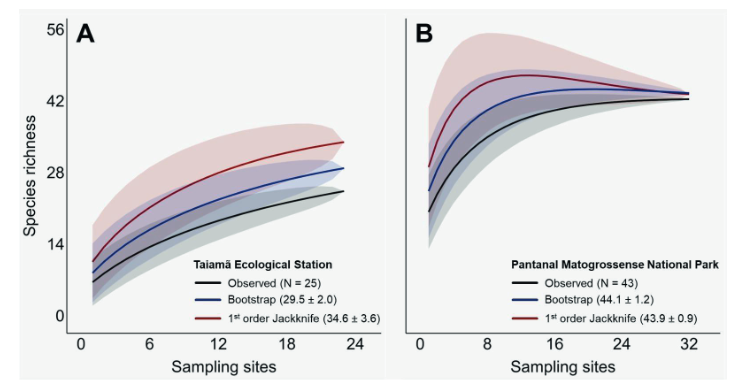
The environmental DNA (eDNA) metabarcoding is a methodology that, from environmental samples such as soil, water, air, and others, enables the simultaneous identification of multiple species, thus allowing for large-scale mapping of biological diversity in a specific study area. Due to its non-invasive sampling approach, where species are detected from the traces they leave in the environment, eliminating the need to isolate and capture organisms, eDNA metabarcoding emerges as a valuable tool in conservation strategies. This study aims to explore the use of eDNA methodology for biodiversity monitoring and environmental impact assessment caused by the 2020 megafire in the Pantanal of Brazil, focusing on vertebrates. Environmental samples were collected at two protected areas and their surrounding areas, Taiamã Ecological Station (TES) and Pantanal Matogrossense National Park (PMNP), Mato Grosso, Brazil. We identified in TES, 27 mammals, 56 fishes, 12 birds, 4 amphibians, and 4 reptiles, while in PMNP, 43 mammals, 45 fishes, 126 birds, 19 amphibians, and 11 reptiles. Soil sampling proved to be more efficient compared to water sampling: 26 species were exclusively identified in soil samples, while 9 were attributed to water samples. Here, we demonstrated that the primer 12SV5 only a superior efficacy in identifying mammal and herpetofauna species compared to the other markers used (16Smam and MiBird). Moreover, we confirmed the complementary role of eDNA alongside camera trapping, and its advantage to estimate species richness with a single field expedition. We stress the need to optimize sample collection methods for the target group and to reduce the influence of contamination and water flow. This study highlights the importance of eDNA methodology as a crucial tool for biodiversity monitoring and environmental impact assessment, enabling rapid access to biodiversity and long-term monitoring.
Downloads
Referências
1. Libonati R, Belém LBC, Rodrigues JA, Santos FLM, Sena CAP, Pinto MM, Carvalho IA. Sistema ALARMES – Alerta de área queimada Pantanal, situação final de 2020; 2021. [acesso em 08 abr 2024]; Disponível em https:// www. researchgate.net/publication/ 35010 3205_ Nota_Tecnica_ 012021_ LASA- UFRJ_ Queimadas_Pantanal_2020?channel=doi&linkId=6051109d92851cd8ce483fb1&showFulltext= true.
2. Menezes LS, de Oliveira AM, Santos FLS, Russo A, de Souza RAF, Roque FO, Libonati R. Lightning patterns in the Pantanal: Untangling natural and anthropogenic-induced wildfires. Science of the Total Environment. 2022; 820: 153021. doi: 10.1016/j.scitotenv.2022.153021. DOI: https://doi.org/10.1016/j.scitotenv.2022.153021
3. Berlinck CB, Lima LHA, Pereira AMM, Carvalho Jr EAR, Paula RC, Tomas WM, Morato RG. The Pantanal is on fire and only a sustainable agenda can save the largest wetland in the world. Braz. J. Biol. 2021; 82: e244200. doi: 10.1590/1519-6984.244200. DOI: https://doi.org/10.1590/1519-6984.244200
4. Libonati R, DaCamara CC, Peres FL, de Carvalho LAS, Garcia L.C. Rescue Brazil’s burning Pantanal wetlands. Nature. 2020; 588: 217-219. DOI: https://doi.org/10.1038/d41586-020-03464-1
5. Marengo JA, Cunha AP, Cuartas LA, Leal KRD, Broedel E, Seluchi ME et al. Extreme drought in the Brazilian Pantanal in 2019-2020: Characterization, causes and impacts. Front. Water. 2021; 3: 639204. doi: 10.3389/frwa.2021.639204. DOI: https://doi.org/10.3389/frwa.2021.639204
6. Garcia LC, Szabo JK, Roque FO, Pereira AMM, da Cunha CN, Damasceno-Júnior GA et al. Record-breaking wildfires in the world’s largest continuous tropical wetland: Integrative Fire Management is urgently needed for both biodiversity and humans. J. Environ. Manag. 2021; 293: 112870. doi: 10.1016/j.jenvman.2021.112870. DOI: https://doi.org/10.1016/j.jenvman.2021.112870
7. Libonati R, Geirinhas JL, Silva PS, Russo A, Rodrigues JA, Belém LBC et al. Assessing the role of compound drought and heatwave events on unprecedent 2020 wildfire in the Pantanal. Environ. Res. Lett. 2022; 17: 015005. doi: 10.1088/1748-9326/ac462e. DOI: https://doi.org/10.1088/1748-9326/ac462e
8. Lorenz C, Libonati R, Belém LBC, Oliveira A, Chiaravalloti RM, Nunes AV, Batista EKL, Fernandes GW, Chiaravalloti-Neto F, Damasceno-Junior GA, Berlinck CN, Roque FO. Wildfire and smoke association with COVID cases in the Pantanal wetland, Brazil. Public Health 2023; 225: 311e319. doi: 10.1016/j.puhe.2023.10.032. DOI: https://doi.org/10.1016/j.puhe.2023.10.032
9. Ribeiro DB, Pereira AMM. Solving the problem of wildfires in the Pantanal Wetlands. Perspectives in Ecology and Conservation 2023; 21(4): 271-273. doi: 10.1016/j.pecon.2023.10.004. DOI: https://doi.org/10.1016/j.pecon.2023.10.004
10. Magioli M, Lima LHA, Villela PMS, Sampaio R, Bonjorne L, Ribeiro RLA et al. Forest type modulates mammalian responses to megafires. Scientific Reports 2024; 14: 13538. doi: 10.1038/s41598-024-64460-3. DOI: https://doi.org/10.1038/s41598-024-64460-3
11. Berlinck CN, Lima LHA, Carvalho Junior EAR. Historical survey of research related to fire management and fauna conservation in the world and in Brazil. Biota Neotropica. 2021; 21(3): e20201144. doi: 10.1590/1676-0611-BN-2020-1144. DOI: https://doi.org/10.1590/1676-0611-bn-2020-1144
12. Tomas WM, Berlinck CN, Chiaravalloti RM, Faggioni GP, Strüssmann C, Libonati R et al. Distance sampling surveys reveal 17 million vertebrates directly killed by the 2020’s wildfires in the Pantanal, Brazil. Sci Rep. 2021; 11: 23547. doi: 10.1038/s41598-021-02844-5. DOI: https://doi.org/10.1038/s41598-021-02844-5
13. Thomsen PF, Willerslev E. Environmental DNA – An emerging tool in conservation for monitoring past and present biodiversity. Biological Conservation. 2015; 183: 4-18. DOI: https://doi.org/10.1016/j.biocon.2014.11.019
14. Taberlet P, Bonin A, Zinger L, Coissac E. Environmental DNA. Vol 1. Oxford: University Press; 2018. DOI: https://doi.org/10.1093/oso/9780198767220.003.0001
15. Bohmann K, Evans A, Gilbert MT, Carvalho GR, Creer S, Knapp M, Yu DW, de Bruyn M. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol Evol. 2014; 29(6): 358-67. doi: 10.1016/j.tree.2014.04.003. DOI: https://doi.org/10.1016/j.tree.2014.04.003
16. Valentini A, Taberlet P, Miaud C, Civade R, Herder J, Thomsen PF et al. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Molecular Ecology. 2016; 25(4): 929-942. doi: 10.1111/mec.13428. DOI: https://doi.org/10.1111/mec.13428
17. Carvalho CS, de Oliveira ME, Rodriguez-Castro KG, Saranholi BH, Galetti PM Jr. Efficiency of eDNA and iDNA in assessing vertebrate diversity and its abundance. Mol Ecol Resour. 2022; 22(4): 1262-1273. doi: 10.1111/1755-0998.13543. DOI: https://doi.org/10.1111/1755-0998.13543
18. Sales NG, Kaizer MdC, Coscia I, Perkins JC, Highlands A, Boubli JP, Magnusson WE, Da Silva MNF, Benvenuto C, Mcdevitt AD. Assessing the potential of environmental DNA metabarcoding for monitoring Neotropical mammals: a case study in the Amazon and Atlantic Forest, Brazil. Mam Rev. 2020; 50: 221-225. https://doi.org/10.1111/mam.12183.
19. Deiner K, Walser JC, Mächler E, Altermatt F. Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA. Biol. Conserv. 2015; 183: 53-63. DOI: https://doi.org/10.1016/j.biocon.2014.11.018
20. Sigsgaard E, Carl H, Møller P, Thomsen P. Monitoring the near-extinct European weather loach in Denmark based on environmental DNA from water samples. Biological Conservation. 2015; 183: 46-52. doi: 10.1016/j.biocon.2014.11.023. DOI: https://doi.org/10.1016/j.biocon.2014.11.023
21. Longmire JL, Maltbie M, Baker RJ. Use of “lysis buffer” in DNA isolation and its implications for museum collections. Museum of Texas Tech University. 1997; 163: 1-3. DOI: https://doi.org/10.5962/bhl.title.143318
22. Leempoel K, Hebert T, Hadly EA. A comparison of eDNA to camera trapping for assessment of terrestrial mammal diversity. Proceedings of the Royal Society B: Biological Sciences. 2020; 287: 1918. doi: 10.1098/rspb.2019.2353.
23. Taberlet P, Prud’Homme SM, Campione E, Roy J, Miquel C et al. Soil sampling and isolation of extracellular DNA from large amount of starting material suitable for metabarcoding studies. Mol Ecol. 2012; 21(8): 1816-1820. doi: 10.1111/j.1365-294X.2011.05317.x. DOI: https://doi.org/10.1111/j.1365-294X.2011.05317.x
24. Riaz T, Shehzad W, Viari A, Pompanon F, Taberlet P, Coissac E. ecoPrimers: inference of new DNA barcode markers from whole genome sequence analysis. Nucleic Acids Res. 2011; 39(21): e145. doi: 10.1093/nar/gkr732. DOI: https://doi.org/10.1093/nar/gkr732
25. Taylor PG. Reproducibility of ancient DNA sequences from extinct Pleistocene fauna. Molecular biology and evolution. 1996; 13(1): 283-285. DOI: https://doi.org/10.1093/oxfordjournals.molbev.a025566
26. Ushio M, Murata K, Sado T, Nishiumi I, Takeshita M, Iwasaki W, Miya M. Demonstration of the potential of environmental DNA as a tool for the detection of avian species. Sci Rep. 2018; 8: 4493. doi: 10.1038/s41598-018-22817-5. DOI: https://doi.org/10.1038/s41598-018-22817-5
27. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016; 13(7): 581-583. doi: 10.1038/nmeth.3869.
28. Mahé S. v2: highly-scalable and high-resolution amplicon clustering. CALLAHAN, Benjamin J et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nature methods. 2016; 13(7): 581-583. DOI: https://doi.org/10.1038/nmeth.3869
29. TEAM Network. Terrestrial Vertebrate Protocol Implementation Manual, v. 3.1. Tropical Ecology, Assessment and Monitoring Network, Center for Applied Biodiversity. 2011. Available from: https://catalog.ipbes.net/system/assessment/136/references/files/385/original/TEAMTerrestrialVertebrate-PT-EN-3.1.pdf?1353346399.
30. Ahumada JA, Fegraus E, Birch T, Flores N, Kays R, O’Brien TG et al. Wildlife Insights: A Platform to Maximize the Potential of Camera Trap and Other Passive Sensor Wildlife Data for the Planet. Environmental Conservation. 2020; 47(1): 1-6. doi: 10.1017/S0376892919000298. DOI: https://doi.org/10.1017/S0376892919000298
31. MMA – Ministério do Meio Ambiente. 2022. Portaria MMA no 148, de 7 de junho de 2022. Altera os Anexos da Portaria no 443, de 17 de dezembro de 2014, da Portaria no 444, de 17 de dezembro de 2014, e da Portaria no 445, de 17 de dezembro de 2014, referentes à atualização da Lista Nacional de Espéc. Diário Oficial da União 108: 74. (Acessed July 20, 2022). doi: 10.22420/rde.v11i20.774 DOI: https://doi.org/10.22420/rde.v11i20.774
32. Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P et al. 2022. vegan: Community Ecology Package. R package version 2. 6-4, <https://CRAN.R-project.org/package=vegan>.
33. Chiarello AG. Density and population size of mammals in remnants of Brazilian Atlantic Forest. Conserv. Biol. 2000; 14: 1649-1657. DOI: https://doi.org/10.1046/j.1523-1739.2000.99071.x
34. Emmons LH, Feer F. 1997. Neotropical Rainforest Mammals: A Field Guide. University of Chicago Press, Chicago.
35. Abreu EF, Casali D, Costa-Araújo R, Garbino GST, Libardi GS, Loretto D et al. 2023. Lista de Mamíferos do Brasil (2023-1) [Data set]. Zenodo. doi: 10.5281/zenodo.10428436
36. Guedes TB, Entiauspe-Neto OM, Costa HC. 2023. Lista de répteis do Brasil: atualização de 2022. doi: 10.5281/zenodo.7829013.
37. Segalla M, Berneck B, Canedo C, Caramaschi U, Cruz CAG; Garcia PCA et al. List of Brazilian Amphibians. Herpetologia Brasileira. 2021; 10(1): 121-216. doi: 10.5281/zenodo.4716176.
38. Falk-petersen J, Bøhn T, Sandlund OT. On the numerous concepts in invasion biology. Biol. Invasions. 2006; 8: 1409-1424. doi: 10.1007/s10530-005-0710-6. DOI: https://doi.org/10.1007/s10530-005-0710-6
39. Kvist S. Barcoding in the dark? A critical view of the sufficiency of zoological DNA barcoding databases and a plea for broader integration of taxonomic knowledge. Mol Phylogenet Evol. 2013; 69(1): 39-45. doi: 10.1016/j.ympev.2013.05.012. DOI: https://doi.org/10.1016/j.ympev.2013.05.012
40. Sales NG, Kaizer MdC, Coscia I, Perkins JC, Highlands A, Boubli JP et al. Assessing the potential of environmental DNA metabarcoding for monitoring Neotropical mammals: a case study in the Amazon and Atlantic Forest, Brazil. Mam Rev. 2020; 50: 221-225. doi: 10.1111/mam.12183. DOI: https://doi.org/10.1111/mam.12183
41. Saranholi BH, Rodriguez-Castro KG, Carvalho CS, Chahad-Ehlers S, Gestich CC, Andrade SCS, Freitas PD, Galetti PMJr. Comparing iDNA from mosquitoes and flies to survey mammals in a semi-controlled Neotropical area. Molecular Ecology Resources. 2023; 23(8): 1790-1799. doi: 10.1111/1755-0998.13851. DOI: https://doi.org/10.1111/1755-0998.13851
42. Saranholi BH, França FM, Vogler AP, Barlow J, Vaz De Mello FZ, Maldaner ME, Galetti PMJr. Testing and optimizing metabarcoding of iDNA from dung beetles to sample mammals in the hyperdiverse Neotropics. Molecular Ecology Resources. 2024. doi: 10.1111/1755-0998.13961. DOI: https://doi.org/10.1111/1755-0998.13961
43. Massey AL, Bronzoni RVdeM, da Silva DJF, Allen JM, de Lázari PR, dos Santos-Filho M, Canale GR, Bernardo CSS, PeresCA, Levi T. Invertebrates for vertebrate biodiversity monitoring: Comparisons using three insect taxa as iDNA samplers. Molecular Ecology Resources. 2022; 22(3): 962-977. doi: 10.1111/1755-0998.13525. DOI: https://doi.org/10.1111/1755-0998.13525
44. Champlot S, Berthelot C, Pruvost M, Bennett EA, Grange T, Geigl EM. An efficient multistrategy DNA decontamination procedure of PCR reagents for hypersensitive PCR applications. PLoS One. 2010; 5(9): e13042. DOI: https://doi.org/10.1371/journal.pone.0013042
45. Boessenkool S, Epp LS, Haile J, Bellemain E, Edwards M, Coissac E, Willerslev E, Brochmann C. Blocking human contaminant DNA during PCR allows amplification of rare mammal species from sedimentary ancient DNA. Molecular Ecology. 2012; 21(8): 1806-1815. doi: 10.1111/j.1365-294x.2011.05306.x. DOI: https://doi.org/10.1111/j.1365-294X.2011.05306.x
46. Bardales R, Boron V, Viana DFP, Sousa LL, Droge E, Porfirio G et al. Neotropical mammal responses to megafires in the Brazilian Pantanal. Glob Change Biol. 2024; 30: e17278. 10.1111/gcb.17278. DOI: https://doi.org/10.1111/gcb.17278
47. Wilcox TM, McKelvey KS, Young MK, Engkjer C, Lance RF, Lahr A, Eby LA, Schwartz MK. Parallel, targeted analysis of environmental samples via high-throughput quantitative PCR. Environmental DNA 2020; 2(4): 544-553. doi: 10.1002/edn3.80. DOI: https://doi.org/10.1002/edn3.80
48. Dejean T, Valentini A, Miquel C, Taberlet P, Bellemain E, Miaud C. Improved detection of an alien invasive species through environmental DNA barcoding: the example of the American bullfrog Lithobates catesbeianus. J. Appl. Ecol. 2012; 49: 953-959. doi: 10.1111/j.1365-2664.2012.02171.x. DOI: https://doi.org/10.1111/j.1365-2664.2012.02171.x
49. Thomsen PF, Kielgast J, Iversen LL, Møller PR, Rasmussen M, Willerslev E. Detection of a Diverse Marine Fish Fauna Using Environmental DNA from Seawater Samples. PLOS ONE. 2012; 7(8): e41732. doi: 10.1371/journal.pone.0041732. DOI: https://doi.org/10.1371/journal.pone.0041732
50. Thomsen PF, Kielgast J, Iversen LL, Wiuf C, Rasmussen M, Gilbert MTP, Orlando L, Willerslev E. Monitoring endangered freshwater biodiversity using environmental DNA. Mol. Ecol. 2012; 21: 2565-2573. doi: 10.1111/j.1365-294X.2011.05418.x. DOI: https://doi.org/10.1111/j.1365-294X.2011.05418.x
51. Tsuji S, Ushio M, Sakurai S, Minamoto T, Yamanaka H. Water temperature-dependent degradation of environmental DNA and its relation to bacterial abundance. PLOS ONE. 2017; 12: e0176608. DOI: https://doi.org/10.1371/journal.pone.0176608
52. Amaral CRL, Chaves ACS, Borges Júnior VNT, Pereira F, Silva BM et al. Amphibians on the hotspot: Molecular biology and conservation in the South American Atlantic Rainforest. PLOS ONE. 2019; 14(10): e0224320. doi: 10.1371/journal.pone.0224320. DOI: https://doi.org/10.1371/journal.pone.0224320
53. West KM, Heydenrych M, Lines R, Tucker T, Fossette S, Whiting S, Bunce M. Development of a 16S metabarcoding assay for the environmental DNA (eDNA) detection of aquatic reptiles across northern Australia. Marine and Freshwater Research. 2021; 74: 432-440. doi: 10.1071/MF20288. DOI: https://doi.org/10.1071/MF20288
Publicado
Edição
Seção
Licença
Copyright (c) 2024 Os autores mantêm os direitos autorais de seus artigos sem restrições, concedendo ao editor direitos de publicação não exclusivos.

Este trabalho está licenciado sob uma licença Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Os artigos estão licenciados sob uma licença Creative Commons Atribuição-NãoComercial-SemDerivações 4.0 Internacional (CC BY-NC-ND 4.0). O acesso é livre e gratuito para download e leitura, ou seja, é permitido copiar e redistribuir o material em qualquer mídia ou formato.










